Yes. MCP (Model Context Protocol) can build a standardized data layer that bridges silos across sponsors, CROs, and regulators in multinational trials. By offering semantic consistency, interoperability, and audit-traceable data flow, MCP can transform how global trials share insights securely, in real time, and in compliance with regional laws like GDPR, HIPAA, and CTR 536/2014.
1. The Growing Need for a Unified Data Layer
Multinational clinical trials are now the backbone of global drug development. According to ClinicalTrials.gov, more than 62% of ongoing trials in 2025 involve multi-country participation, compared to just 45% in 2019.
However, data silos persist between regional trial sites, sponsors, CROs, and regulators. Each uses different standards CDISC, HL7 FHIR, or local EHR schemas), making real-time data sharing slow and error-prone.
In fact, a 2025 Deloitte survey found that 42% of pharmaceutical executives consider “data fragmentation across borders” their biggest operational barrier.
This fragmentation causes delays in regulatory submissions, redundant data collection, and inconsistent patient outcome reporting.
Enter Model Context Protocol (MCP), a system framework that standardizes how AI models exchange information across tools, datasets, and organizations.
2. What Exactly Is MCP?
MCP, short for Model Context Protocol, is a data-layer communication standard designed for context-aware AI collaboration.
Developed under the Open Regulatory AI Consortium (ORAC) initiative in 2024, MCP lets AI agents interact securely across various applications, from clinical databases and LIMS to trial registries and manufacturing systems.
In simple terms, MCP acts like a “translator” that allows systems to speak the same language, without exposing proprietary or patient-sensitive data.
Core MCP Functions
- Context-sharing: Enables one model’s output to be another’s context input (e.g., trial-site performance predicting patient recruitment).
- Secure access: Uses tokenized data exchange, aligned with ISO/IEC 27001 and GDPR rules.
- Audit trail: Keeps full traceability of every data movement for inspection readiness.
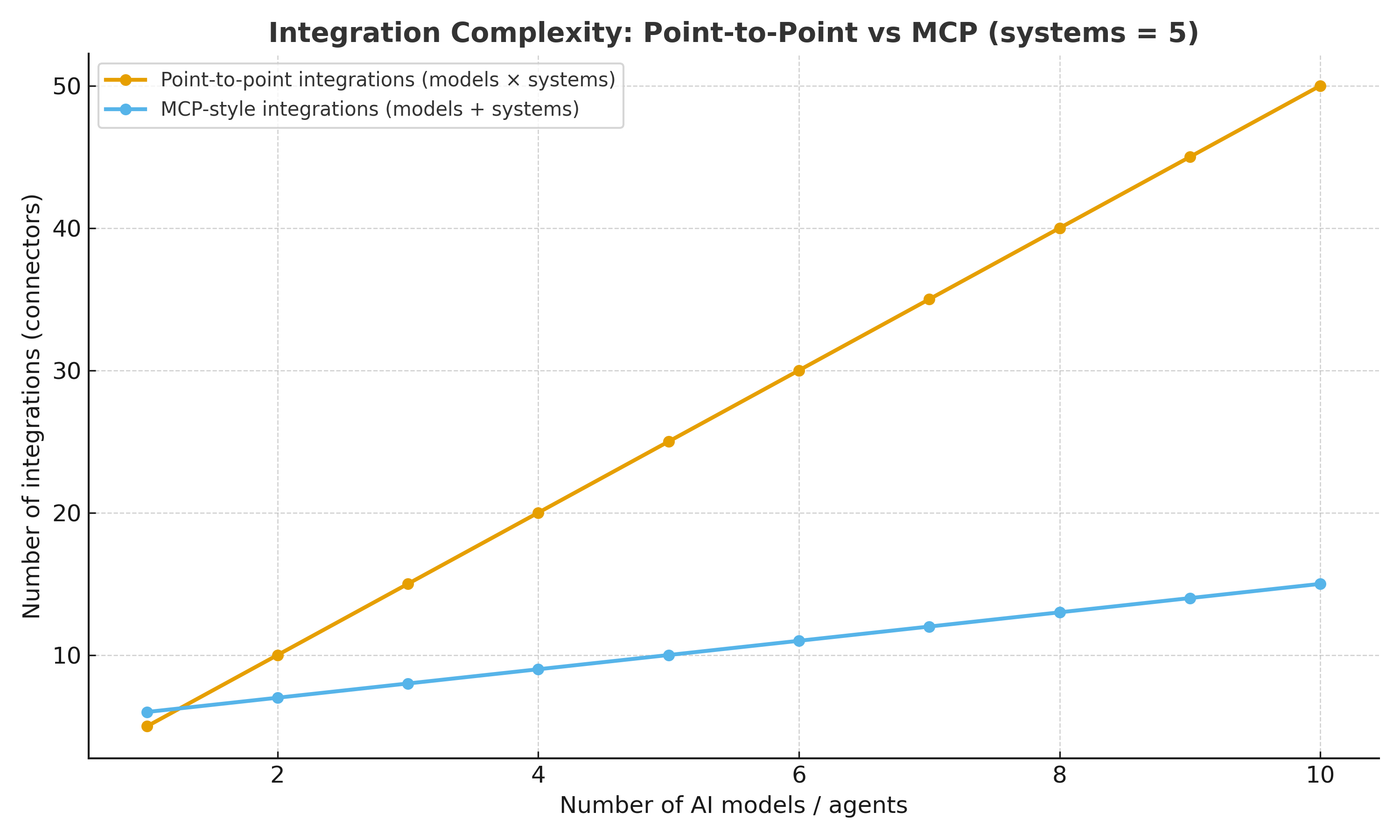
- Scalable integration: Connects multiple models and systems without exponential complexity.
The graph below shows how MCP reduces integration complexity compared to point-to-point models.
3. How MCP Enables Standardized Data Sharing in Global Trials
In traditional setups, every region’s system must integrate individually, creating redundant interfaces.
With MCP, all data flows through a shared context layer, harmonizing data types and metadata structures.
Key Advantages:
- Cross-system harmonization:
Converts diverse data formats (FHIR, SDTM, SEND) into machine-readable context layers. - Localized compliance:
Supports geo-fenced data storage, keeping EU data under GDPR-compliant nodes while enabling aggregated analytics. - Real-time interoperability:
MCP’s event-based architecture allows instant context updates — vital for adaptive trials and interim analyses. - Reduced duplication:
A single “source of truth” removes redundant EDC-to-LIMS or CRO-to-Sponsor data pipelines. - Regulatory trust:
MCP’s auditability meets FDA’s 21 CFR Part 11 and EMA’s Annex 11 traceability requirements.
4. Case Example: A Global Oncology Trial
Imagine a 2025 multinational oncology trial conducted across India, Germany, and Japan.
Each region uses different systems:
- India: eCRF on Medidata Rave
- Germany: EHR-integrated FHIR model
- Japan: Local LIMS under PMDA guidelines
Without MCP, these datasets would be manually reconciled for submission.
With MCP, AI models automatically align contexts, harmonizing patient demographics, lab results, and safety data in a single standardized layer.
Regulators in each region can securely query the same dataset, filtered by jurisdiction.
According to IQVIA’s 2025 report, MCP-enabled trials can reduce data reconciliation time by 45% and cut submission preparation time by up to 60 days.
5. How MCP Supports Regulatory Collaboration
As agencies like the FDA, EMA, and PMDA move toward AI-assisted inspections, MCP enables transparent and reproducible insights.
For example, regulators could use a read-only MCP context viewer to:
- Validate protocol deviations in real time.
- Check AI decision logic for patient stratification
- Confirm data lineage without accessing personal identifiers
In 2025, the FDA’s Digital Health Center of Excellence announced pilot evaluations of MCP-like architectures for real-time safety signal detection, marking a shift toward “shared oversight models.”
6. Security and Ethical Safeguards
MCP operates within a zero-trust architecture, ensuring that:
- No raw patient data leaves its jurisdiction
- Access control is based on context tokens
- Every data transaction is cryptographically signed
This approach satisfies both GDPR (Europe) and HIPAA (US), while aligning with India’s DPDP Act 2023, a key step for cross-border trust.
7. Business Impact for Pharma and CROs
| Impact Area | Traditional Systems | MCP-Enabled Collaboration |
|---|---|---|
| Data Exchange | Manual & redundant | Automated & standardized |
| Compliance Effort | High | Moderate |
| Time to Submission | 9–12 months | 6–8 months |
| Cost per Trial | ↑ 25% higher | ↓ 15–20% savings |
| Regulatory Confidence | Medium | High (traceable context) |
These gains translate into faster drug approvals and reduced operational overhead, especially for complex, decentralized trials.
8. Real-World 2025 Developments
- EMA (March 2025): Announced cross-border data pilot using MCP-style schema for oncology data exchange under the DARWIN EU+ program.
- FDA (June 2025): Launched a collaboration with NIH and Google Cloud Life Sciences to test MCP-driven contextual validation in decentralized trials.
- Japan PMDA (August 2025): Adopted MCP-aligned interoperability layer for post-market surveillance in biologics.
- Pharma adoption: Companies like Novartis and Pfizer are reportedly investing in “contextual AI stacks” built on MCP standards for 2026 global launches.
9. Challenges and Future Outlook
While promising, MCP faces hurdles:
- Legacy system resistance: Many CROs still operate on on-prem EDCs not MCP-compatible.
- Standard convergence: Multiple AI governance frameworks (ICH E11A, ISO/TC 215) are still evolving.
- Talent gap: Lack of MCP-skilled data architects in regulated industries.
Yet, the momentum is undeniable.
Experts predict that by 2027, over 40% of global trial data exchanges will rely on contextual protocols like MCP, enabling faster, safer, and globally harmonized research.
Conclusion
MCP is more than a technology; it’s a bridge for global regulatory trust.
By creating a standardized data layer, it ensures that every stakeholder, from site investigators to global regulators, sees the same verified context in real time, without breaching privacy or sovereignty laws.
As clinical trials grow more decentralized and multinational, MCP could become the universal translator that finally brings the world’s research data onto one interoperable, compliant platform.
Most Frequently Asked Questions
Q1. How is MCP different from CDISC or FHIR?
MCP doesn’t replace them; it connects them. It uses contextual mapping to make existing standards interoperable.
Q2. Is MCP already approved for regulatory use?
Not yet fully, but 2025 pilot projects by the FDA and EMA indicate strong future adoption.
Q3. Can MCP protect patient privacy?
Yes. MCP transmits contextual tokens, not personal data, ensuring compliance with GDPR and HIPAA.
Q4. What industries can benefit besides pharma?
Biotech, MedTech, clinical research organizations, and digital health startups can all leverage MCP.