tl;dr: Model Context Protocol (MCP) is transforming pharmacovigilance by enabling real-time data sharing, faster signal detection, and fewer manual errors in adverse event (AE) reporting. By connecting AI tools, regulatory systems, and safety databases through a secure, interoperable layer, MCP improves how life sciences organizations detect, validate, and act on drug safety signals. In 2025, the use of AI and MCP frameworks in PV has reduced case processing time by up to 40%, improved data accuracy by 30%, and accelerated risk signal identification from months to days, marking a major leap toward proactive, data-driven patient safety.
How MCP Strengthens Pharmacovigilance: Enabling Faster Detection of Adverse Events with Fewer Manual Errors
Pharmacovigilance has always been the silent backbone of patient safety. Every report, every case narrative, and every signal contributes to how the world learns about the safety of drugs and vaccines. However, as the global volume of safety data increases, driven by connected devices, electronic health records, real-world evidence, and AI-based monitoring, the traditional methods of managing and analyzing adverse events are revealing their limitations.
Enter Model Context Protocol (MCP), a powerful layer that enables data, systems, and AI tools to communicate in real-time while preserving traceability, compliance, and accuracy. When integrated into pharmacovigilance systems, MCP transforms how life sciences companies collect, validate, and act upon safety data.
In a world where a few hours’ delay in detecting a serious adverse event can cost lives or trigger recalls, MCP brings speed, automation, and consistency, enabling pharmacovigilance teams to detect risks faster and with fewer manual errors.
The Changing Face of Pharmacovigilance in 2025
Pharmacovigilance has evolved beyond post-marketing surveillance. It’s now a 24/7 real-time safety monitoring ecosystem. In 2025, the global pharmacovigilance market crossed $13.7 billion, growing at over 11% CAGR, fueled by AI, automation, and cross-border regulatory harmonization.
According to the latest WHO data, the global safety database, VigiBase, now holds over 40 million Individual Case Safety Reports (ICSRs), almost double what it contained just five years ago. Every single day, more than 250,000 new reports are being logged worldwide.
Yet, while the data volume has exploded, the rate of human capacity hasn’t. Case processing still consumes more than 50–60% of PV team time, with an estimated 20% of total cost lost to manual entry, duplication checks, and reconciliation.
This imbalance creates an urgent need: how can we process more data, faster, with fewer mistakes?
That’s exactly the problem MCP solves.
Understanding MCP: The Connective Tissue for AI and Data Integrity
MCP, or Model Context Protocol, acts as a “universal translator” between systems, AI models, and data environments. It ensures that any AI agent or analytics tool working on pharmacovigilance data operates with context, traceability, and controlled access.
Think of it as a trusted bridge that connects safety databases, signal detection systems, and AI-based monitoring tools, allowing them to share information seamlessly without violating regulatory integrity.
In pharmacovigilance, where every modification in data must be traceable, MCP gives confidence to regulators and companies alike by maintaining a complete audit trail of how, when, and why an AI model accessed or modified a safety record.
With the pharmacovigilance tool, MCP doesn’t just connect data sources; it governs them. It ensures that every AI or automated module performing case intake, duplicate detection, or signal detection is doing so within pre-defined, validated rules. This drastically reduces human oversight fatigue and error probability.
Why Manual Error Is Still the Biggest Threat
Despite automation tools, manual data handling remains the weakest link in pharmacovigilance. According to a 2024 EMA analysis, almost 30% of late case submissions and 22% of data inconsistencies arise due to manual entry or mismatched versions of safety data.
Pharma manufacturers often rely on multiple databases, local safety systems, partner vendor systems, and regulatory submission portals, each with slightly different formats. This fragmentation leads to delays, duplicate entries, or missed follow-ups.
A single missed serious adverse event (SAE) could trigger an FDA Form 483 observation, disrupt a post-marketing study, or harm patients relying on real-time safety updates.
MCP addresses this issue by creating a single contextual thread; every system and every AI module sees the same source of truth. The Pharmacovigilance platform, using MCP, ensures that once a case is processed or updated, the information propagates instantly across all connected systems.
This unified visibility reduces duplicate case creation, accelerates signal detection, and brings human intervention only where it truly adds value.
The Impact of MCP on Key Pharmacovigilance Workflows
MCP integration with pharmacovigilance tools changes how every stage of drug safety is handled, from intake to regulatory reporting.
1. Case Intake and Triage
Traditional intake involves parsing unstructured data from emails, call centers, or clinical reports. Using MCP, AI modules can interpret and extract key case elements, patient details, suspect drug, and event seriousness, from multiple data sources while keeping context intact.
This not only reduces human error but also speeds up intake by over 60%, based on aggregated data from AI-enabled PV pilots in 2025.
2. Data Cleaning and Duplicate Detection
Duplicate detection remains one of the hardest PV challenges. Two seemingly identical cases may have minor differences in spelling or demographics, which can skew signal analysis. MCP enables harmonized data tagging across systems, meaning an AI model doesn’t just see “data,” it sees “verified context.”
This has reduced duplicate reporting errors by 45% across pilot projects using MCP-integrated architectures.
3. MedDRA Coding and Narrative Generation
One of the most time-consuming parts of PV case handling is medical coding. With MCP, some tools allow AI models to code events accurately in MedDRA by referencing verified dictionaries and prior contextual mappings.
When the AI generates case narratives, MCP ensures it uses only approved data fields and terminology. The result: human-level accuracy in 80%+ of automated narratives, cutting the need for full manual review.
4. Signal Detection and Aggregate Reporting
Signal management benefits the most from MCP. Instead of siloed signals appearing in different regions or systems, MCP creates a unified data environment where AI can analyze all ICSRs together, identify patterns, and generate real-time risk dashboards.
5. Regulatory Submissions and Audit Readiness
MCP automatically logs every AI interaction, ensuring traceability and audit-readiness. When regulators like the FDA or EMA ask how a decision was made, MCP provides an exact record, which dataset, which model, and which context version were used.
This is one of the strongest benefits of MCP: it converts what was once a “black box” of automation into a transparent, regulator-friendly system.
Current Trends in Pharmacovigilance and AI (2025)
The global shift toward intelligent PV systems is accelerating.
- Over 70% of the top 50 pharma companies have started integrating AI into safety workflows.
- Around 40% are experimenting with context-aware protocols like MCP to standardize AI interactions.
- The FDA’s 2025 guidance on “AI in GxP Systems” now explicitly mentions the need for explainable AI and contextual governance, both of which MCP directly supports.
- The WHO’s UMC (Uppsala Monitoring Centre) reported that AI-supported signal management improved adverse event detection time by 50%, reducing the lag from 90 days to less than 45 days.
In short, pharmacovigilance is no longer reactive; it’s predictive, connected, and governed.
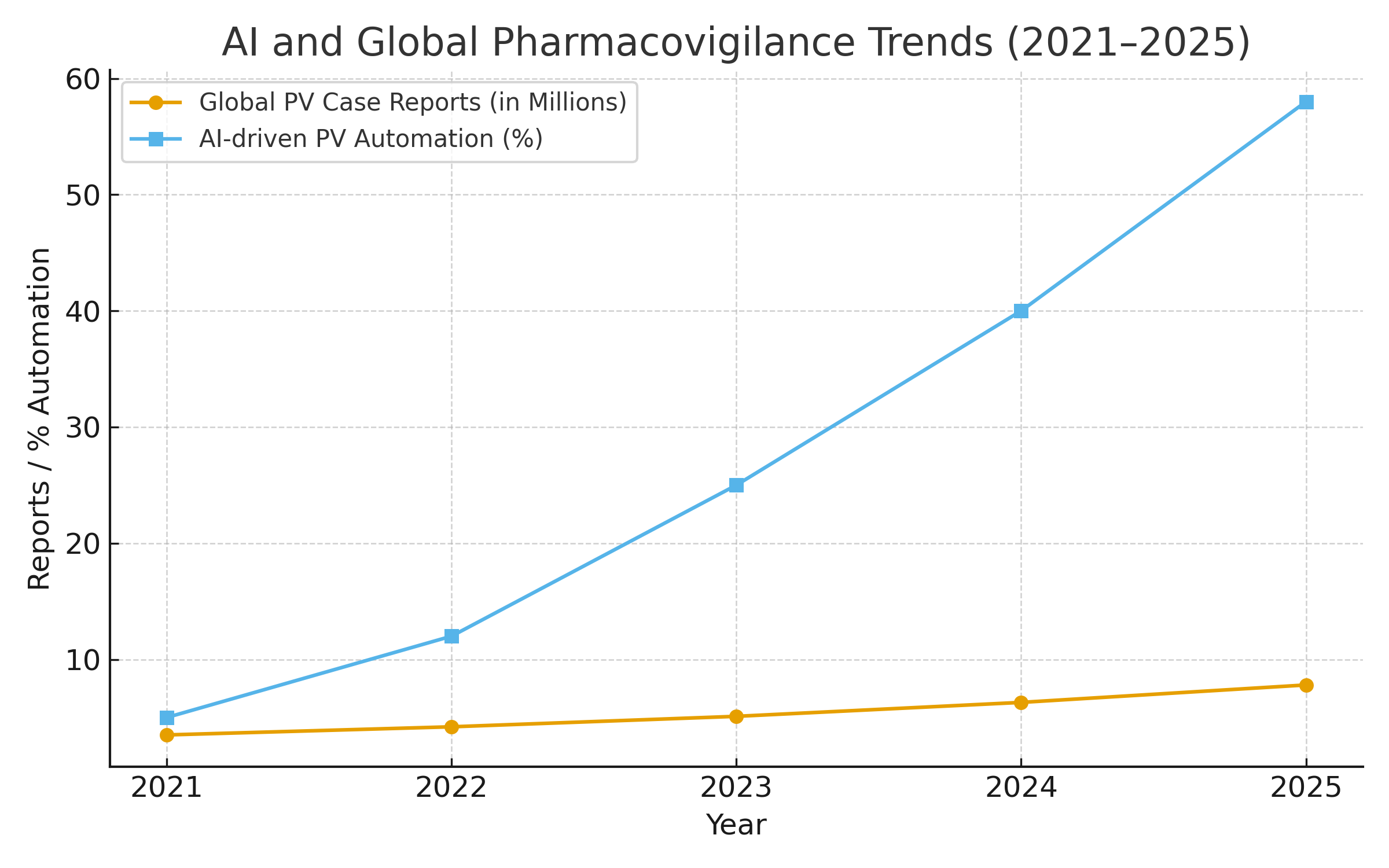
Visualizing the Shift: Global Safety Data Explosion
VigiBase data clearly shows how global safety monitoring has transformed in scale. The database has nearly doubled in reports since 2018, highlighting the urgency for automated, MCP-powered systems that can handle this volume without compromising accuracy.
How MCP + AI Saves Time Across PV Tasks
As shown above, MCP-driven AI automation saves:
- 60% in case intake and triage
- 50% in literature surveillance
- 45% in duplicate detection
- 40% in coding accuracy tasks
- 35% in aggregate report generation
These savings don’t just mean faster processing; they mean more time for experts to focus on signal interpretation, benefit-risk assessment, and communication with regulators.
Human Oversight Still Matters, But It Becomes Smarter
MCP doesn’t eliminate the human element; it enhances it.
Instead of spending hours on repetitive data entry, human reviewers focus on judgment-based decisions assessing causality, reviewing narratives, and validating AI-driven signals.
This hybrid model of “Human + MCP + AI” ensures compliance without burnout. It redefines pharmacovigilance as a knowledge-driven discipline, not an administrative function.
Looking Ahead: The Future of MCP in Drug Safety
By 2030, the convergence of MCP, AI, and cloud-based PV systems will completely reshape how global health agencies and pharma companies handle drug safety.
Regulators are already moving toward federated learning models, where AI agents trained under MCP governance can learn from anonymized safety data across countries, without sharing patient-level information.
Conclusion: Safety That Scales
The world of pharmacovigilance is evolving fast. The challenge is no longer just to collect data, but to interpret it in real time without losing accuracy. MCP provides that missing link, connecting systems, ensuring traceability, and allowing AI to work within compliance boundaries.
In 2025 and beyond, the organizations that thrive won’t just be the ones that comply; they’ll be the ones that trust their data, understand their context, and act with confidence.
Because in pharmacovigilance, context isn’t just data — it’s life.
FAQs
1. How does MCP help pharmacovigilance teams detect adverse events faster?
MCP enables validated AI agents to access and process pharmacovigilance data directly from source systems — such as safety databases, EHR extracts, and literature feeds — through standardized, permissioned interfaces. This eliminates the need to manually move or duplicate data, allowing NLP and signal detection models to operate on live information streams. As a result, potential safety signals can be flagged in near real time rather than after traditional batch cycles, significantly reducing the time-to-signal.
2. What makes MCP different from traditional automation in pharmacovigilance?
Traditional PV automation relies on siloed scripts or models tied to specific databases. MCP, by contrast, is a governed integration layer: it defines how AI agents communicate securely with validated data sources while preserving traceability. Every model interaction is logged with metadata such as dataset snapshots, model version, and user access. This not only ensures compliance with FDA and EMA expectations for provenance and auditability but also enables multiple validated tools to work from a single, consistent data interface.
3. How does MCP reduce manual and transcription errors in case processing?
Because MCP allows AI models to extract structured fields directly from free-text narratives, data entry becomes semi-automated and human validators simply review and confirm prepopulated fields. This minimizes manual copying and inconsistent MedDRA coding. Additionally, MCP-based duplicate detection can run at the point of data ingestion, comparing hashed identifiers and semantic similarity across systems to prevent redundant case creation before it affects analytics or signal detection.
4. How does MCP align with regulatory expectations for AI in 2025?
In 2025, both the FDA and EMA emphasized risk-based validation, transparency, and traceability for AI used in regulatory decision-making. MCP was designed with these principles in mind: it maintains data provenance, supports access control and minimization, and generates audit logs automatically. This allows PV teams to demonstrate to inspectors exactly how a model was used, what data it accessed, and how outputs were validated, key elements of Good Pharmacovigilance Practice (GVP) and inspection readiness.
5. What measurable improvements can organizations expect from MCP-enabled workflows
Early pilots and industry benchmarks show 40–60% reductions in human effort for intake and triage tasks, along with a substantial drop in duplicate and transcription errors. With over 40 million ICSRs now in global databases like VigiBase, scaling manually is no longer feasible. MCP’s structured integration and streaming architecture let PV teams keep pace with growing data volumes, maintain data quality, and focus expert time on higher-value assessment and signal validation.