tl;dr: In a world where regulatory scrutiny continues to tighten, life sciences companies can’t afford to fall behind on compliance. Whether you’re preparing for an FDA inspection or trying to stay ahead of regulatory trends, one name keeps surfacing among compliance teams: Atlas Compliance. But what exactly is Atlas Compliance, and how can it help your organization?

1. What is Atlas Compliance?
Atlas Compliance is a regulatory intelligence platform designed to help pharmaceutical, biotech, and medical device companies navigate global compliance demands. It centralizes data from the FDA and other regulatory bodies, offering:
- Real-time access to inspection records (e.g., Form 483s, EIRs, Warning Letters)
- AI-driven insights on compliance risks
- Searchable databases across multiple agencies
- Custom alerts based on your company, products, or facilities
Atlas is not just a database. It’s a compliance decision-making tool built to empower teams with actionable data and predictive insights.
“Think of Atlas as your regulatory assistant who never sleeps.”
2. Who Should Use Atlas Compliance?
Atlas is designed for:
- Regulatory Affairs Managers
- Quality Assurance Teams
- Pharmacovigilance Departments
- CRO/CMO Partners
- Compliance Officers
Any team responsible for regulatory submissions, inspections, and quality operations will find value in the platform.
Even CEOs and board members can benefit by using Atlas to:
- Track trends in regulatory enforcement
- Understand risk exposure across global sites
- Benchmark performance against competitors
3. What Problems Does It Solve?
Many teams face these compliance challenges:
- Scattered data across PDFs, spreadsheets, and emails
- No real-time alerts on inspection findings
- Inability to benchmark competitors’ audit outcomes
- Poor visibility into the FDA’s evolving focus areas
Atlas solves these by offering:
- Centralized compliance data in one searchable dashboard
- Live alerts on new inspections or enforcement actions
- AI summaries of historical trends
- Benchmarking tools for competitive risk comparison
With Atlas, your team spends less time digging through documents and more time preparing smart responses.
4. How Is It Different from Other Compliance Tools?
Most compliance systems are either:
- Document repositories (not dynamic)
- Audit tools focused only on internal processes
- Consultant-driven (costly, manual updates)
Atlas sets itself apart by offering
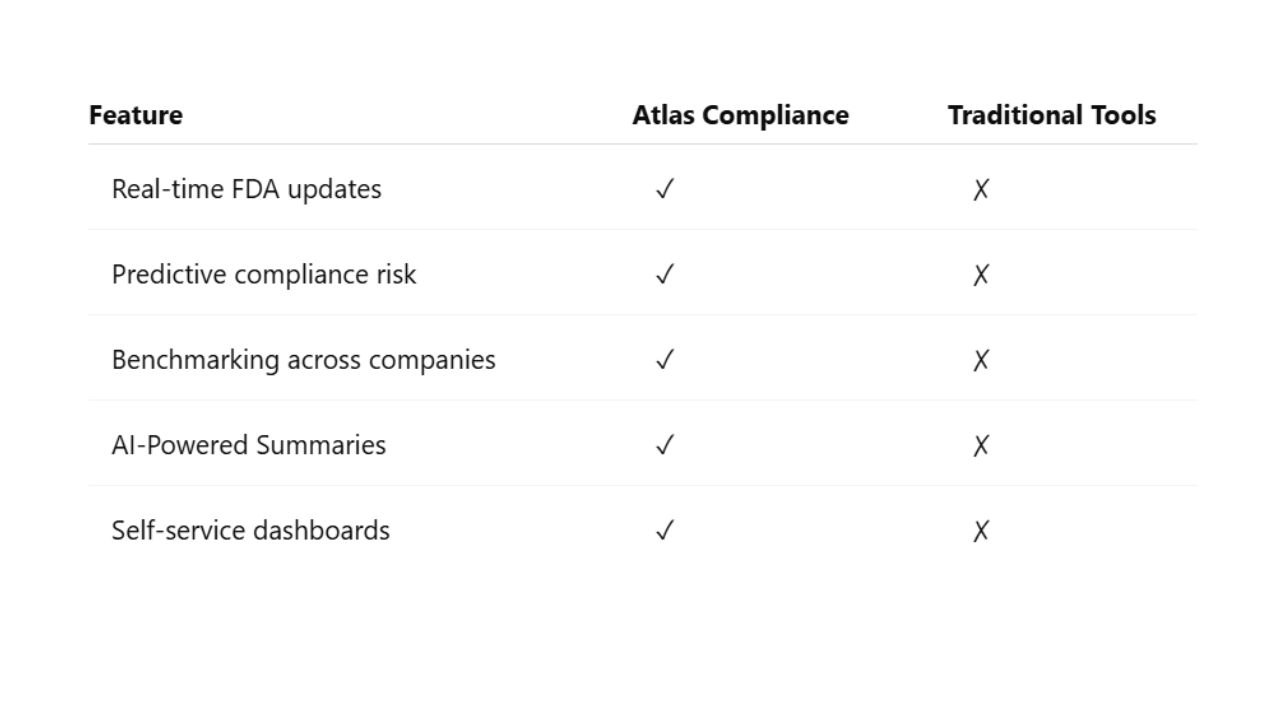
Atlas is built with usability and agility in mind. It’s plug-and-play for QA teams.
5. What Type of Data Does Atlas Offer?
Atlas houses a massive database including:
- Form 483s: Official inspection observations
- EIRs: Establishment Inspection Reports
- Warning Letters: Regulatory enforcement actions
- Import Alerts: Impact on international operations
- Inspection Histories: Across companies, sites, and countries
- Real-time Updates: On newly published enforcement data
Each document is enriched with:
- AI-powered summaries
- Keyword tagging (CAPA, data integrity, cGMP, etc.)
- Risk-level scoring
6. Is Atlas Updated in Real Time?
Yes, and this is one of its strongest advantages.
Unlike static databases, Atlas is connected via smart crawlers and APIs to:
- FDA’s inspection and enforcement portals
- EMA, MHRA, and other international bodies
You get real-time push notifications for:
- Any new Form 483 linked to your competitor
- Warning Letters related to your product category
- Inspection updates in your geographical focus areas
This helps teams respond before issues escalate.
7. How Secure is Atlas Compliance?
Atlas is built on enterprise-grade cloud infrastructure and complies with major security standards:
- SOC 2 Type II compliant
- End-to-end data encryption
- Role-based access controls
- Multi-factor authentication
- Continuous vulnerability monitoring
All user activities are audit-tracked, which is essential for regulated environments.
Your regulatory data stays safe, controlled, and audit-ready.
8. Can Atlas Help During FDA Inspections?
Absolutely. In fact, it was designed with that moment in mind.
During inspections, Atlas can:
- Provide instant historical data on similar inspection outcomes
- Help prepare responses using past language from other Form 483s
- Identify FDA focus areas based on recent enforcement actions
- Benchmark your site’s inspection frequency vs. competitors
Case Example: A sterile drug manufacturer used Atlas to prepare for an unannounced FDA visit and avoided 3 major citations by pre-empting known risk trends.
9. What Support or Onboarding Does Atlas Offer?
Onboarding is swift and supported by:
- Dedicated implementation specialists
- Step-by-step video walkthroughs
- Personalized training sessions for each team
- 24/7 support for technical issues or regulatory queries
Atlas is also user-friendly, meaning most teams are up and running within 2–3 days.
We signed up and were getting compliance alerts within 48 hours, no IT team needed.” – Director of QA, Mid-size Pharma
10. What is the ROI of Using Atlas Compliance?
Return on investment comes from:
- Reduced inspection risk: Proactive insights help avoid costly observations
- Faster audit readiness: Saves hours of manual research
- Smarter strategic decisions: Use enforcement trends to guide compliance priorities
- Benchmarking: Identify how you’re doing vs. peers
Sample ROI Metrics:
- 30% faster audit response times
- 2–4x increase in inspection preparedness
- 50% reduction in manual document analysis time
In short, Atlas helps your team become compliance agile and future-ready.
If you’re tired of chasing regulatory changes, searching scattered inspection reports, or manually tracking FDA trends, Atlas offers a smarter way forward.
It’s more than a tool, it’s your strategic partner in compliance.
Want to see how Atlas can support your next inspection or internal audit? Book a free demo now and stay one step ahead of compliance risk.