The Model Context Protocol (MCP) is a new standard that allows AI models to connect easily with data, systems, and tools. In pharma R&D, this opens major opportunities in drug discovery, clinical trial design, manufacturing integration, regulatory compliance, and knowledge management. With good data quality, governance, and validation, it can shorten timelines, reduce manual work, and improve audit-readiness. But success depends on the underlying systems and discipline, not just the protocol alone.
1. What is MCP, and why does it matter to pharma R&D
What is MCP?
- MCP stands for Model Context Protocol. It is an open standard that defines how AI models (especially large language models or agents) interact with external tools, databases, workflows, and systems consistently.
- Think of MCP like a “universal plug” for AI: instead of each system building a custom connector for every tool, MCP gives a standard way for AI and systems to communicate.
- Technically, MCP uses a client-server style architecture: the AI host (client) sends requests to MCP servers, which expose tools or data sources, using JSON-RPC messages and defined schemas.
Why does it matter for pharma R&D?
- Pharma R&D encompasses various systems, including experiment logs, assay databases, clinical trial data, manufacturing systems (LIMS and MES), and regulatory documents. These systems are usually separate.
- AI models can bring value (e.g., summarising literature, designing assays, predicting risks) but only when they can access the right data and tools in context. MCP helps make that access easier and more reliable.
- For regulated industries (such as pharma), traceability, auditability, and reproducibility are crucial. MCP helps because the protocol defines how calls to tools/data are logged and structured.
- With MCP, AI-driven workflows become more realistic: an agent can pull internal assay data, access external literature, query manufacturing logs, and propose actions, all within a compliant framework.
2. High-level map of key MCP use-cases in pharma R&D
Here are major use-cases where MCP brings value, organised by domain and estimated relative impact. Later, we will deep-dive into each.
| Drug discovery & target identification | Using data to optimise protocol, patient selection, simulations, and site feasibility. |
| Clinical trial design & execution | Connecting AI agents to MES/LIMS, real-time data streams, and predictive maintenance. |
| Manufacturing & shop-floor integration | Helping scientists find prior art, summarise literature, and link to internal data. |
| Regulatory compliance & audit‐ready workflows | Ensuring AI workflows have full traceability, automating document generation. |
| Knowledge management & literature triage | Automating evidence-collection for deviations, faster investigations, and fewer manual steps. |
| Quality assurance & batch release support | Automating evidence-collection for deviations, faster investigations, fewer manual steps. |
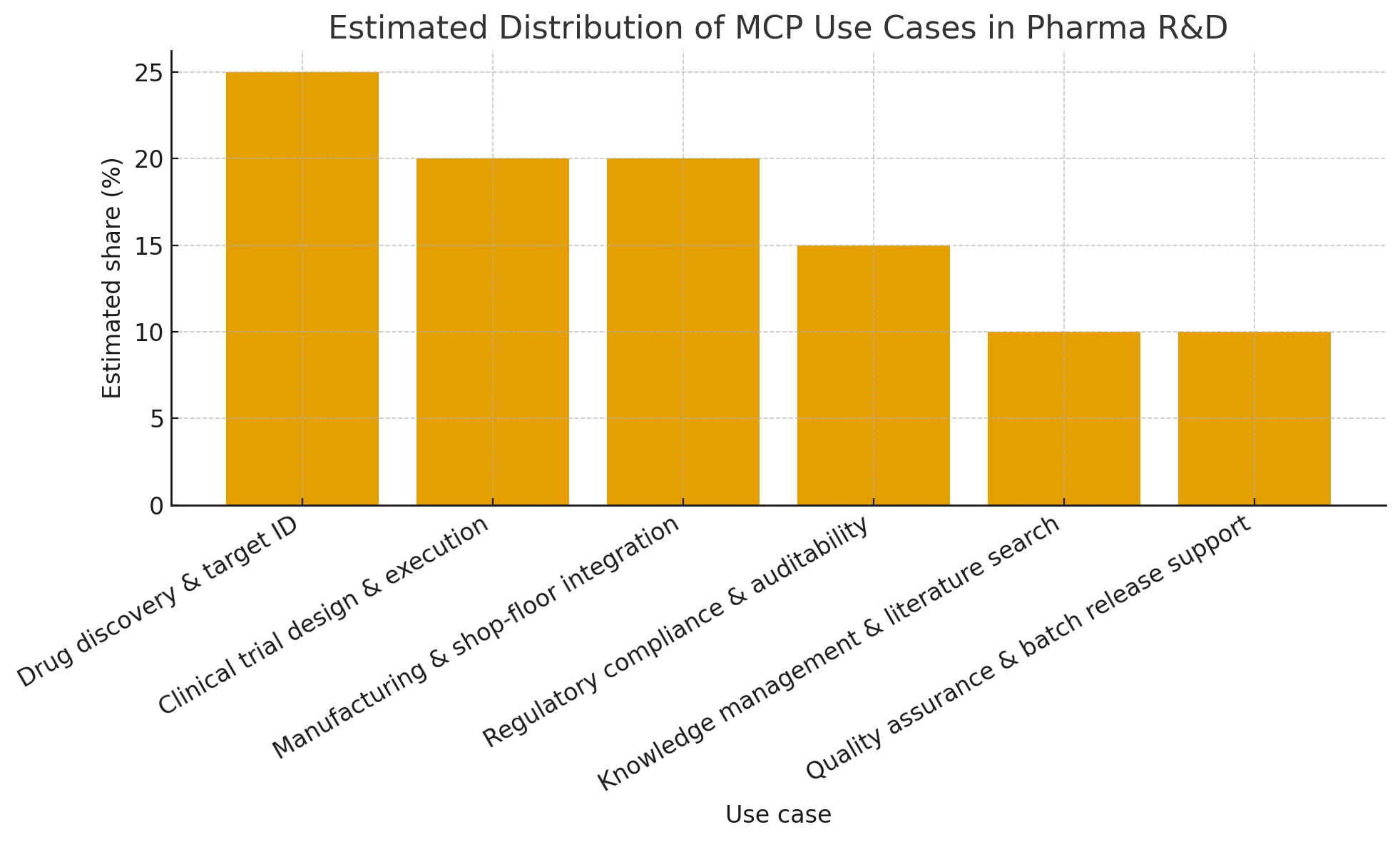
Estimated distribution (for planning focus):
- Drug discovery ~ 25%
- Clinical trials ~ 20%
- Manufacturing/shop-floor ~ 20%
- Regulatory/compliance ~ 15%
- Knowledge management ~ 10%
- QA/batch release ~ 10%
(Note: these numbers are estimates derived from current industry writing, for prioritisation guidance.)
3. Deep dives: Use-cases with examples, benefits, and metrics
a) Drug discovery & target identification
What’s enabled
- AI agents connected via MCP can run pipelines: fetch internal assay results, call virtual screening tools, query cheminformatics libraries, and propose new leads.
- The agent can maintain provenance: which data sources were queried, which tools were used, and log outcomes for audit and reuse.
Why it matters
- Early-stage drug discovery is costly and slow. Faster iteration means faster decision-making and lower cost of failure.
- With MCP, integration effort is lower, meaning more time is spent on science, less on plumbing.
- Example: a team can ask, “What compounds have shown activity on kinase X in our database?” The MCP-enabled agent pulls the data, runs similarity searches, ranks candidates, and logs the path.
Metrics & impact
- Time per lead-generation cycle could drop from months to weeks in well‐equipped settings (though exact numbers vary).
- Better reuse of internal data, fewer duplicated experiments.
b) Clinical trial design & execution
What’s enabled
- Agents can query trial data lakes, real-world data (RWD), site feasibility tools, enrolment dashboards, and simulate outcomes.
- Protocol amendments can be modelled: an AI agent could generate draft amendment documents, justify them based on data, and log which data sources were used.
Why it matters
- Many trial delays are caused by patient recruitment issues, site selection, and protocol amendments.
- Faster, more data-driven design lowers risk and cost.
- Example: trial team asks for “projected recruitment in region A for inclusion criteria X.” The agent uses EHR data, filters sites, estimates timeline, and produces a ranked list of sites.
Metrics & impact
- Reduction in recruitment time (site-specific)
- Fewer protocol amendments after start
- Faster site activation cycles
c) Manufacturing & shop-floor integration
What’s enabled
- An agent connected via MCP pulls instrument telemetry, MES logs, LIMS assay results, environmental conditions, and then helps identify drift, root causes, or predict maintenance needs.
- It can also fetch SOPs, batch records, calibration logs, and suggest corrective actions.
Why it matters
- In pharma manufacturing, real-time data integration is expensive and complex. MCP simplifies it.
- Improved efficiency, fewer batch failures, better compliance.
- Example: during a long stability test, variability appears. The agent pulls calibration history, environmental logs, recent operator changes and suggests likely causes.
Metrics & impact
- Reduced the mean time to detect/resolve equipment issues
- Improved overall equipment effectiveness (OEE)
- Fewer rejected batches
d) Regulatory compliance & audit-ready workflows
What’s enabled
- MCP ensures all tool calls and data accesses are logged with structured metadata (which system, which data, when). This helps with audit trails.
- Agents can automate drafting of regulatory documents (e.g., stability summaries, batch release reports) by pulling validated data from ELN/LIMS/QMS systems.
Why it matters
- Regulators increasingly expect transparency and reproducibility. Any AI-driven recommendation must show where the data came from and how the recommendation was generated.
- By design, MCP supports that traceability.
- Example: QA team triggers the agent to prepare a deviation root cause summary. The agent logs all data pulls, tool calls and produces a report ready for review.
Metrics & impact
- Less time spent assembling evidence for audits.
- Higher traceability coverage
- Reduced compliance risk
e) Knowledge management & literature triage
What’s enabled
- Agents query internal experiment results, external literature (PubMed, patents), link results, extract themes, and summarise findings.
- Scientists can ask, “Has anyone tried molecule Y for indication Z?” and the agent returns internal and external evidence.
Why it matters
- Scientists often waste time replicating studies or missing prior art. Faster knowledge retrieval accelerates innovation.
- Example: A researcher proposes a new target. The agent searches literature, finds 15 papers, plus internal assay data, summarises gaps, and suggests experiments.
Metrics & impact
- Reduced literature review time
- Higher reuse of internal data
- Lower duplication of experiments
f) Quality assurance & batch release support
What’s enabled
- Agents collect evidence for batch release: instrument logs, calibration records, deviations, operator notes, and environmental logs. Then they generate a first-draft root cause and evidence package.
- Batch release teams can flag exceptions; the system proposes investigation paths.
Why it matters
- QA teams in pharma spend many hours gathering and reviewing evidence. This reduces manual work and improves speed.
- Example: After an out-of-specification (OOS) result, the agent pulls all relevant records, correlates environmental shifts, calibration history, suggests a likely cause, and prepares an investigation summary.
Metrics & impact
- Faster investigation closure
- Fewer manual hours for evidence collection
- Improved compliance timelines
4. Implementation: What you must plan & manage
Even though MCP simplifies integration, you cannot ignore fundamentals. Key areas:
Data governance & quality
- The best MCP agent is useless if the underlying data are inconsistent, outdated, or untrusted.
- Ensure canonical schemas, versioning, metadata, semantic tagging, and strong ETL.
- Audit trails: know when data came in, how it was processed.
Access control & security
- Permit only least-privilege access. Agents often need wide data access, ensure correct permissions, session isolation, and tokenisation.
- Monitor for misuse or unintended tool calls. One security paper found many vulnerabilities in open MCP servers.
- Regularly update and patch MCP servers, monitor identity fragmentation (a known risk), and ensure accountability.
Validation and reproducibility
- In pharma, workflows driving decisions must be validated and documented (GxP mindset). For MCP-driven agents: validate connectors, provenance logging, and decision logic.
- Maintain runbooks: what tools were called, what data, what logic. Ensure reproducibility of agent outcomes.
Human-in-the-loop and supervision
- For high-risk decisions (e.g., batch release, regulatory submission), keep humans in the loop initially.
- Use the agent for suggestions, not final sign-off until reliability is proven.
- Build escalation paths: when uncertain, hand off to a human expert.
Monitoring, metrics & continuous improvement
- Track tool-call volumes, latency, error rates, and outcomes of agent suggestions vs. human baseline.
- Use dashboards to correlate agent recommendations with downstream success (e.g., fewer trial delays, fewer batch failures).
- Use feedback to refine models, data, and workflows.
Phased rollout
Avoid big-bang. Use a staged approach:
- Phase 0 (0-3 months): Map systems (ELN, LIMS, MES, data lakes), identify one pilot use-case with high value but moderate complexity (e.g., knowledge management).
- Phase 1 (3-9 months): Build a pilot with a human-in-loop. Track metrics.
- Phase 2 (9-18 months): Scale to more complex domains (manufacturing, clinical trials) and add governance.
- Phase 3 (>18 months): Fully operational, monitored, and validated for regulated workflows.
5. Limitations and risks
It’s important to be realistic and aware of what MCP does not fix.
- Dependency on data & tool quality: MCP provides connectivity and standardisation, but it does not automatically improve data quality or tool logic.
- Not a “black box” fix: You still need governance, validation, domain expertise, and oversight.
- Regulatory uncertainty: Although traceability is improved, regulators may still question how AI-agent-derived decisions were made. Provide full evidence and human oversight.
- Security & vendor lock-in risks: MCP is new, ecosystems are evolving. Some MCP servers may have vulnerabilities (e.g., tool poisoning) or insufficient maintenance.
- Change management: People must adopt new workflows. Just deploying technology without training and culture means lower adoption.
6. Summary and next steps for R&D leaders
Key take-aways
- MCP is a standard protocol that helps AI agents access data and tools in a consistent way, making them more powerful and enterprise-ready.
- In pharma R&D, the high-value use-cases span discovery, clinical trials, manufacturing, compliance, knowledge management, and QA.
- Success depends on strong foundations: data quality, security, validation, human oversight, and phased deployment.
- With the right approach, firms can shorten timelines, reduce manual work, improve audit-readiness, and advance innovation.
Immediate next steps
- Map your organisation’s major data systems and evaluate readiness: which data lakes, LIMS, ELN, MES exist, and what is their quality?
- Select one pilot use case with clear business value and moderate complexity, for example, literature triage or internal knowledge retrieval.
- Define governance framework: who owns data, who approves agent recommendations, and what audit logs are needed.
- Create baseline metrics: current time for process X, manual hours, error rates, and you will measure improvement.
- Build a cross-functional team: include R&D scientists, data engineers, QA/regulatory, IT/security.
- Monitor continuously: instrument tool calls, outcomes, user feedback, and refine.
Conclusion
The Model Context Protocol (MCP) is a significant enabler for AI in enterprise and life sciences. For pharma R&D, it offers a path to move from “AI experiments” to operational AI-agent workflows that touch discovery, trials, manufacturing, compliance, and knowledge management. But technology alone is not enough. Strong data, governance, validation, and people are crucial. Firms that adopt a phased, governed path will gain advantages: faster insights, lower risk, and more reproducible outcomes.
Most frequently asked questions related to the subject.
Q1. Is MCP ready for regulated pharma use?
A: The protocol is technically mature in many use cases for data access. However, in a regulated pharma environment, you still need validation, audit logs, qualified data systems, and human oversight. MCP helps but does not replace those requirements.
Q2. What is a good use case to start with?
A: Knowledge management or literature triage is a good start: it has relatively low regulatory risk, shorter cycle time, and can yield value early.
Q3. Will adopting MCP remove the need for data engineers?
A: No. You still need engineers, but their work shifts: instead of building many custom connectors, they focus on data quality, schema mapping, governance, and observability of agent workflows.
Q4. How does MCP help with audit and compliance?
A: Because MCP defines and logs the tool calls, data sources, context, and results, the workflow becomes traceable. That supports audit readiness, reproducibility, and regulatory documentation.
Q5. What are the big security concerns with MCP?
A: If improperly implemented, agents might access data they shouldn’t, or a tool call might trigger unexpected actions. Identity fragmentation, insufficient access controls, and a lack of governance are known issues. Monitoring, least-privilege access, and patching are essential.